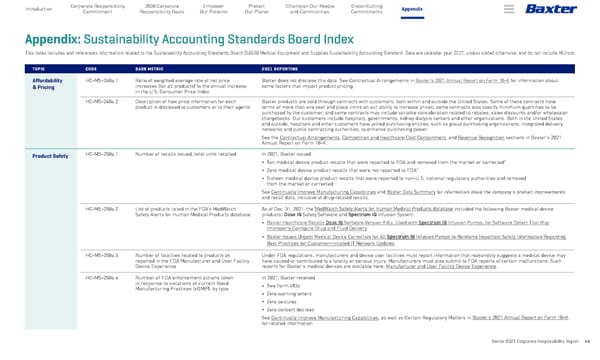
Baxter 2021 Corporate Responsibility Report 59 Appendix 2030 Corporate Responsibility Goals Corporate Responsibility Commitment Empower Our Patients Protect Our Planet Champion Our People and Communities Introduction Cross-Cutting Commitments Appendix: Sustainability Accounting Standards Board Index This index includes and references information related to the Sustainability Accounting Standards Board (SASB) Medical Equipment and Supplies Sustainability Accounting Standard. Data are calendar year 2021, unless stated otherwise, and do not include Hillrom. TOPIC CODE SASB METRIC 2021 REPORTING Affordability & Pricing HC-MS-240a.1 Ratio of weighted average rate of net price increases (for all products) to the annual increase in the U.S. Consumer Price Index Baxter does not disclose this data. See Contractual Arrangements in Baxter’s 2021 Annual Report on Form 10-K for information about some factors that impact product pricing. HC-MS-240a.2 Description of how price information for each product is disclosed to customers or to their agents Baxter products are sold through contracts with customers, both within and outside the United States. Some of these contracts have terms of more than one year and place limits on our ability to increase prices; some contracts also specify minimum quantities to be purchased by the customer; and some contracts may include variable consideration related to rebates, sales discounts and/or wholesaler chargebacks. Our customers include hospitals, governments, kidney dialysis centers and other organizations. Both in the United States and outside, hospitals and other customers have joined purchasing entities, such as group purchasing organizations, integrated delivery networks and public contracting authorities, to enhance purchasing power. See the Contractual Arrangements, Competition and Healthcare Cost Containment, and Revenue Recognition sections in Baxter's 2021 Annual Report on Form 10-K. Product Safety HC-MS-250a.1 Number of recalls issued, total units recalled In 2021, Baxter issued • Ten medical device product recalls that were reported to FDA and removed from the market or corrected 1 • Zero medical device product recalls that were not reported to FDA 1 • Sixteen medical device product recalls that were reported to non-U.S. national regulatory authorities and removed from the market or corrected See Continually Improve Manufacturing Capabilities and Baxter Data Summary for information about the company’s product improvements and recall data, inclusive of drug-related recalls. HC-MS-250a.2 List of products listed in the FDA’s MedWatch Safety Alerts for Human Medical Products database As of Dec. 31, 2021, the MedWatch Safety Alerts for Human Medical Products database included the following Baxter medical device products: Dose IQ Safety Software and Spectrum IQ Infusion System. • Baxter Healthcare Recalls Dose IQ Software Version 9.0.x, Used with Spectrum IQ Infusion Pumps, for Software Defect That May Improperly Configure Drug and Fluid Delivery • Baxter Issues Urgent Medical Device Correction for All Spectrum IQ Infusion Pumps to Reinforce Important Safety Information Regarding Best Practices for Customer-Initiated IT Network Updates HC-MS-250a.3 Number of fatalities related to products as reported in the FDA Manufacturer and User Facility Device Experience Under FDA regulations, manufacturers and device user facilities must report information that reasonably suggests a medical device may have caused or contributed to a fatality or serious injury. Manufacturers must also submit to FDA reports of certain malfunctions. Such reports for Baxter’s medical devices are available here: Manufacturer and User Facility Device Experience . HC-MS-250a.4 Number of FDA enforcement actions taken in response to violations of current Good Manufacturing Practices (cGMP), by type In 2021, Baxter received • Two Form 483s • Zero warning letters • Zero seizures • Zero consent decrees See Continually Improve Manufacturing Capabilities, as well as Certain Regulatory Matters in Baxter’s 2021 Annual Report on Form 10-K for related information.
 Baxter Corporate Responsibility Report Page 58 Page 60
Baxter Corporate Responsibility Report Page 58 Page 60