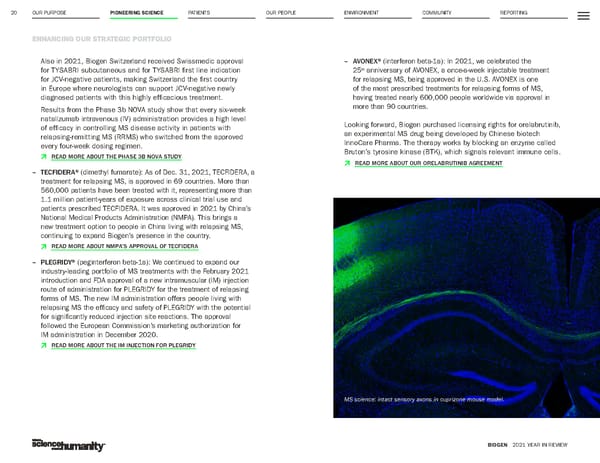
OUR PURPOSE PIONEERING SCIENCE PATIENTS OUR PEOPLE ENVIRONMENT COMMUNITY REPORTING 20 ENHANCING OUR STRATEGIC PORTFOLIO BIOGEN 2021 YEAR IN REVIEW Also in 2021, Biogen Switzerland received Swissmedic approval for TYSABRI subcutaneous and for TYSABRI first line indication for JCV-negative patients, making Switzerland the first country in Europe where neurologists can support JCV-negative newly diagnosed patients with this highly efficacious treatment. Results from the Phase 3b NOVA study show that every six-week natalizumab intravenous (IV) administration provides a high level of efficacy in controlling MS disease activity in patients with relapsing-remitting MS (RRMS) who switched from the approved every four-week dosing regimen. Þ READ MORE ABOUT THE PHASE 3B NOVA STUDY – TECFIDERA® (dimethyl fumarate): As of Dec. 31, 2021, TECFIDERA, a treatment for relapsing MS, is approved in 69 countries. More than 560,000 patients have been treated with it, representing more than 1.1 million patient-years of exposure across clinical trial use and patients prescribed TECFIDERA. It was approved in 2021 by China’s National Medical Products Administration (NMPA). This brings a new treatment option to people in China living with relapsing MS, continuing to expand Biogen’s presence in the country. Þ READ MORE ABOUT NMPA’S APPROVAL OF TECFIDERA – PLEGRIDY® (peginterferon beta-1a): We continued to expand our industry-leading portfolio of MS treatments with the February 2021 introduction and FDA approval of a new intramuscular (IM) injection route of administration for PLEGRIDY for the treatment of relapsing forms of MS. The new IM administration offers people living with relapsing MS the efficacy and safety of PLEGRIDY with the potential for significantly reduced injection site reactions. The approval followed the European Commission’s marketing authorization for IM administration in December 2020. Þ READ MORE ABOUT THE IM INJECTION FOR PLEGRIDY – AVONEX® (interferon beta-1a): In 2021, we celebrated the 25 th anniversary of AVONEX, a once-a-week injectable treatment for relapsing MS, being approved in the U.S. AVONEX is one of the most prescribed treatments for relapsing forms of MS, having treated nearly 600,000 people worldwide via approval in more than 90 countries. Looking forward, Biogen purchased licensing rights for orelabrutinib, an experimental MS drug being developed by Chinese biotech InnoCare Pharma. The therapy works by blocking an enzyme called Bruton’s tyrosine kinase (BTK), which signals relevant immune cells. Þ READ MORE ABOUT OUR ORELABRUTINIB AGREEMENT MS science: intact sensory axons in cuprizone mouse model.
 Biogen Year In Review Page 19 Page 21
Biogen Year In Review Page 19 Page 21