Investor Brief
Invest in a Better Dental Anesthetic — Less painful, faster-acting, more reliable dental injections.
Invest in a Better Dental Anesthetic LESS PAINFUL, FASTER-ACTING, MORE RELIABLE DENTAL INJECTIONS PRIVATE & CONFIDENTIAL
Legal Notice—READ THIS! ASK BPI FOR FURTHER AND UPDATED INFORMATION. DO NOT RELY SOLELY UPON THIS OVERVIEW. This Management Presentation (“Presentation” or “Overview”) and any accompanying materials contain data provided by Balanced Pharma Incorporated (“BPI”). It may not be used for any other purpose except to assist the recipient in determining whether to proceed with further investigation of BPI. THIS INFORMATION IS CONFIDENTIAL AND INTENDED ONLY FOR THE RECIPIENT. By accepting these materials, the recipient agrees to keep the information contained herein confidential and not disclose its contents to third parties or to photocopy, reproduce or distribute to others at any time without the prior written consent of BPI. THIS OVERVIEW DOES NOT CONSTITUTE AN OFFER OR INVITATION FOR THESALE OR PURCHASE OF SECURITIES OR ANY OF THE BUSINESSES OR ASSETS DESCRIBED HEREIN. NOTWITHSTANDING ANY OTHER PROVISION, THIS OVERVIEW IS INTENDED ONLY FOR “ACCREDITED INVESTORS”. Accredited Investors are defined by Rule 501 of Regulation D under the U.S. Securities Act of 1933, as amended, and can bear the risk of investment losses including the potential loss of their entire investment. ANY BPI PRODUCTS MENTIONED ARE IN DEVELOPMENTAL STAGE, HAVE NOTBEEN EVALUATED OR APPROVED BY FDA OR ANY OTHER REGULATORY AUTHORITY, AND ARE NOTFOR SALE IN ANY JURISDICTION OR UNDER ANY CIRCUMSTANCE. NO REPRESENTATION OR WARRANTY, EXPRESSED OR IMPLIED, IS OR WILL BE MADE IN RELATION TO THESE MATERIALS. No responsibility or liability is or will be accepted by BPI or by any of its respective directors, officers, representatives, agents or affiliates as to or in relation to the accuracy or completeness of this overview or any other written or oral information made available to an interested party or its advisors. This Overview and any other materials provided contain forward-looking statements that are only predictions that relate to future events or BPI’s future financial performance, and involve known and unknown risks, uncertainties, and other factors that may cause BPI’s actual results, levels of activity, performance or achievements to be materially different from those implied by these forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as “may,” “will,” “should,” “expects,” “intends,” “plans,” “anticipates,” “believes,” “estimates,” “predicts,” “potential,” “continue,” or the negative of these terms or other comparable terminology. STATEMENTS REGARDING COMPETITORS’ PRODUCTS AND ORGANIZATIONS, WHETHER EXPRESSED OR IMPLIED, ARE ONLY OPINIONS. The recipient should independently verify the validity of any statements about organizations or product attributes, including but not limited to the efficacy, safety, usability, availability, quality, or price of the products or the state of the organization(s) that manufacture, market, or distribute the product(s). View the offering circular at: https://www.sec.gov/Archives/edgar/data/1838860/000110465922092461/tm2223737d1_253g2.htm PRIVATE & CONFIDENTIAL
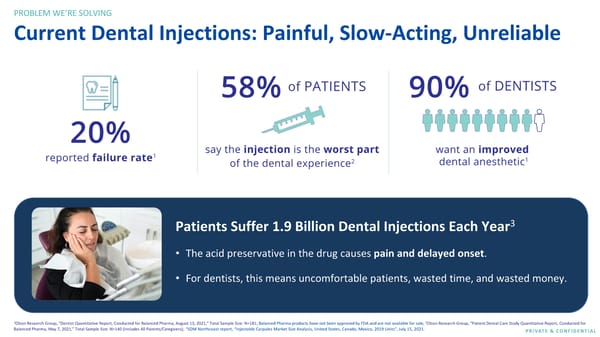
PROBLEM WE’RE SOLVING Current Dental Injections: Painful, Slow-Acting, Unreliable Patients Suffer 1.9 Billion Dental Injections Each Year3 • The acid preservative in the drug causes pain and delayed onset. • For dentists, this means uncomfortable patients, wasted time, and wasted money. 1 2 Olson Research Group, “Dentist Quantitative Report, Conducted for Balanced Pharma, August 13, 2021,” Total Sample Size: N=181; Balanced Pharma products have not been approved by FDA and are not available for sale; Olson Research Group, “Patient Dental Care Study Quantitative Report, Conducted for Balanced Pharma, May 7, 2021,” Total Sample Size: N=140 (Includes 40 Parents/Caregivers); 3SDM Northcoast report, “Injectable Carpules Market Size Analysis, United States, Canada, Mexico, 2019 Units”, July 15, 2021. PRIVATE & CONFIDENTIAL
Introducing Libracaine® Dental1 LIDOCAINE HCL 2% / EPINEPHRINE 1:100,000 / SODIUM BICARBONATE 0.7% Less painful, faster-acting, more reliable2 Supplied in a standard dental cartridge üNo new equipment, no new protocols Patented cartridge technology3 A pH-balanced version of the most often-used anesthetic4 in dentistry üSame safety profile, 24-month shelf life View a 60-second technology demonstration video 1LIBRACAINE HAS NOT BEEN APPROVED BY FDA AND IS NOT AVAILABLE FOR SALE;22018 Meta-analysis: Guo et al from University of Southern California School Of Dentistry concluded: “Buffered lidocaine significantly decreased onset time and injection pain (VAS) compared with non-buffered lidocaine in inferior alveolar nerve block.”; 2017 Clinical Study: Phero et al from University Of North Carolina School Of Dentistry concluded: “Buffered lidocaine reduces the pain on injection with a maxillary field block and results in similar lengths of pulpal anesthesia as non-buffered 2% lidocaine.” 2017 Clinical Study: Warren et al from University Of North Carolina School Of Dentistry concluded: “After mandibular nerve block, buffered 1% lidocaine can produce similar duration of pulpal anesthesia as non-buffered 2% lidocaine and lower pain on injections.”; 2019 Meta-analysis: Kattan et al from University Of Pennsylvania School Of Dentistry concluded: “Buffered local anesthetics have 2.29 times greater likelihood of achieving successful anesthesia [in pulpally involved teeth].” 3BPI owns the following granted patents and pending patent applications: U.S. Patent No. 11,305,064 (issued April 19th, 2022); U.S. Application No. 17/722,016; International PCT Application No. PCT/IB2018/052598; Canadian Application No. 3,111,347 (Canadian National Phase of PCT/IB2018/052598); European Application No. 18897951.2 (European Regional Phase of PCT/IB2018/052598); Japanese Application No. 2020-556351 (Japanese National Phase of PCT/IB2018/052598); Korean Application No. 10-2020-7021685 (Republic of Korea National Phase of PCT/IB2018/052598); U.S. Application No. 16/655,362; U.S. Application No. 63/233,879; BPI also owns one trademark application, U.S. Application No. 88/198,808, pending for the LIBRACAINE mark.; 4SDM Northcoast report, “Injectable Carpules Market Size Analysis, United States, Canada, Mexico, 2019 Units”, July 15, 2021. PRIVATE & CONFIDENTIAL
MARKET RESEARCH Strong Positive Reaction from Dentists 1,2 Are likely to adopt Libracaine Think using Libracaine would increase referrals1,2 Are likely to feature Libracaine on their website 1,2 and in their marketing materials A pH-balanced anesthetic in a standard dental cartridge will be a game changer. “ “ - Jason Goodchild, DMD Dr. Goodchild holds academic appointments at the University of Pennsylvania School of Dental Medicine, Rutgers School of Dental Medicine, and Creighton University School of Dentistry. He has authored more than 50 research papers on the subject of dental pain management. 1 th 2 3 Olson Research Group, “U.S. Dentist Quantitative Report, Conducted For Balanced Pharma, August 13 , 2021,” Total Sample Size: 181 Dentists; LIBRACAINE HAS NOT BEEN APPROVED BY FDA AND IS NOT AVAILABLE FOR SALE; Key-Stone Research, “European Local Anesthetics Market Survey, Conducted For Balanced Pharma, September 20, 2021,” Total Sample Size: 433 Dentists. PRIVATE & CONFIDENTIAL

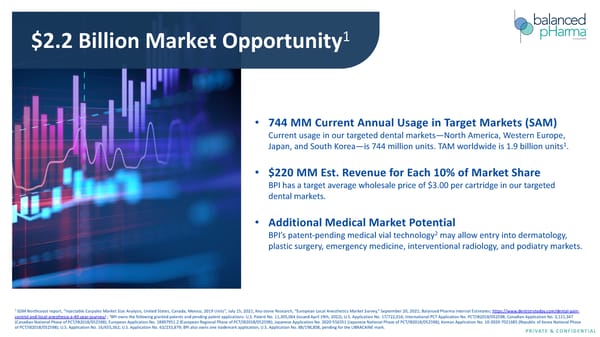
$2.2 Billion Market Opportunity1 • 744 MM Current Annual Usage in Target Markets (SAM) Current usage in our targeted dental markets—North America, Western Europe, 1 Japan, and South Korea—is 744 million units. TAM worldwide is 1.9 billion units . • $220 MM Est. Revenue for Each 10% of Market Share BPI has a target average wholesale price of $3.00 per cartridge in our targeted dental markets. • Additional Medical Market Potential 2 BPI’s patent-pending medical vial technology may allow entry into dermatology, plastic surgery, emergency medicine, interventional radiology, and podiatry markets. 1 SDM Northcoast report, “Injectable Carpules Market Size Analysis, United States, Canada, Mexico, 2019 Units”, July 15, 2021; Key-stone Research, “European Local Anesthetics Market Survey,” September 20, 2021; Balanced Pharma Internal Estimates; https://www.dentistrytoday.com/dental-pain- control-and-local-anesthesia-a-40-year-journey/ ; 2BPI owns the following granted patents and pending patent applications: U.S. Patent No. 11,305,064 (issued April 19th, 2022); U.S. Application No. 17/722,016; International PCT Application No. PCT/IB2018/052598; Canadian Application No. 3,111,347 (Canadian National Phase of PCT/IB2018/052598); European Application No. 18897951.2 (European Regional Phase of PCT/IB2018/052598); Japanese Application No. 2020-556351 (Japanese National Phase of PCT/IB2018/052598); Korean Application No. 10-2020-7021685 (Republic of Korea National Phase of PCT/IB2018/052598); U.S. Application No. 16/655,362; U.S. Application No. 63/233,879; BPI also owns one trademark application, U.S. Application No. 88/198,808, pending for the LIBRACAINE mark. PRIVATE & CONFIDENTIAL
OUR STORY A Mission to Improve Patient Comfort A Need for Less Painful, Faster-Acting Dental Injections Dr. Scott Keadle, a dentist for over 30 years, experienced firsthand the limitations of current dental anesthetic drugs and their inability to provide a fast-acting, reliable, and painless injection. He wanted a better anesthetic for his patients: Less injection pain, less waiting time, and more reliability. A New Anesthetic. Created for Dentists, by Dentists. Dr. Keadle invented patented technology to enable pH-balanced versions of the most common local anesthetics, using a standard syringe and protocol. He then presented the opportunity to a group of dentists who invested over $1 million to help found Balanced Pharma Incorporated (BPI). . Now he leads a team of industry experts who are committed to bringing the first-ever pH-balanced dental anesthetic to market. 1 2 SDM Northcoast report, “Injectable Carpules Market Size Analysis, United States, Canada, Mexico, 2019 Units”, July 15, 2021; BPI owns the following granted patents and pending patent applications: U.S. Patent No. 11,305,064 (issued April 19th, 2022); U.S. Application No. 17/722,016; International PCT Application No. PCT/IB2018/052598; Canadian Application No. 3,111,347 (Canadian National Phase of PCT/IB2018/052598); European Application No. 18897951.2 (European Regional Phase of PCT/IB2018/052598); Japanese Application No. 2020-556351 (Japanese National Phase of PCT/IB2018/052598); Korean Application No. 10-2020-7021685 (Republic of Korea National Phase of PCT/IB2018/052598); U.S. Application No. 16/655,362; U.S. Application No. 63/233,879; BPI also owns one trademark application, U.S. Application No. 88/198,808, pending for the LIBRACAINE mark. Balanced Pharma products have not been approved by FDA and are not available for sale. PRIVATE & CONFIDENTIAL
THE TEAM Experienced Management J. Scott Keadle, DDS JohnSelig Mark Sebree Jim Pellegrini Eric Sherb JasonSuggs ChairmanandCEO Director Director, Chief Development Officer Chief Operating Officer Chief Financial Officer Chief CommunicationsOfficer Founder andCEO at Balanced Pharma, Managing Partner at WaveEdge Capital, Fmr. CEO, Becton Dickinson Rx; 35+ 25+ years in pharma & med device 13 years experience in accounting CEOatOrangeReef,20+years 30+ years as a dentist, practice owner 25+ years in life science investment years in strategy, acquisitions, and strategy, business development, advisory, auditing, and M&A; extensive in brand strategy, communications, andbusinessexecutive banking and M&Apractice operations in the pharma industry engineering & operations experience in financial reporting andtechnology Nita U. Patel, Ph.D. Steve Jensen Alex Sadusky Sara Hanks, Esq. ByronKirkland, Esq. Greg Carlin, Esq. Senior Advisor—CMC Senior Advisor—Regulatory Senior Advisor – Commercial Securities Counsel CorporateCounsel Intellectual Property Counsel SVP, Global Reg. Issues and Head of CMC, EVP, Head of U.S. Regulatory Sciences, CEO, TruBlu Dental; Fmr. Dentsply-Sirona CEO,CrowdCheckLawFirm ManagingPartner, Partner, at ProPharma Group; 30+ years of R&D at ProPharma Group; 22+ years of Sr. Exec.; Fmr. Sr. Ext. Advisor, McKinsey & SmithAndersonLawFirm Meunier, Carlin, & Curfman and pharmaceutical industry experience pharmaceutical industry experience Co. Pharma & Med Products Division PRIVATE & CONFIDENTIAL
THE TEAM Scientific Advisory Board Bishr Al-Dabagh, Larina Chu, DDS, M-PA, CLS Mark Donaldson,Pharm.D. Jay B. Reznick, D.M.D., M.D. John B. Roberson, DMD MD, MBA, FAAD, FACMS Scientific Advisor Scientific Advisor Scientific Advisor Scientific Advisor Scientific Advisor Dentist anesthesiologist serving the greater Associate Principal at Vizient Pharmacy Director of the Southern California Oral & Maxillofacial Surgeon; CEO Board-certified dermatologist Hesperia, California area. Dr. Chu is a Advisory Solutions, 17+ years as a Center for Oral and Facial Surgery andCo-Founder of AAFDO (American Board of Dermatology), professional member of the American recognized expert in dental in Tarzana, California; Diplomate (Accreditation Association For Dental Board of Dental Anesthesiologists, the pharmacology, He holds academic of the American Board of Oral and Oices); CEO and Co-founder of the fellowship-trained dermatologic American Dental Association, the California appointments at the University of Maxillofacial Surgery; the first Institute of Medical Emergency surgeon (American College of Mohs Dental Association and the Tri-County Montana and the Oregon Health & specialist in the U.S. to integrate CBCT Preparedness; author and lecturer; Surgery), and a fellow of the American Sciences University and serves on the and CAD/CAM in his practice; has selected as a CE Leader for Dentistry Academy of Dermatology (AAD). Dr. Dental Society. been published extensively Today for 10 consecutive years Al-Dabaghhas published over 20 peer- editorial board of the Journal reviewed articles and book chapters. of the American Dental Association in dental and medical literature PRIVATE & CONFIDENTIAL
BecomeanInvestor INVEST IN BALANCED PHARMA Better Anesthetic. Better Dentistry. Better Invest. GET IN FOR $4/SHARE Invest.BalancedPharma.com View the offering circular PRIVATE & CONFIDENTIAL


