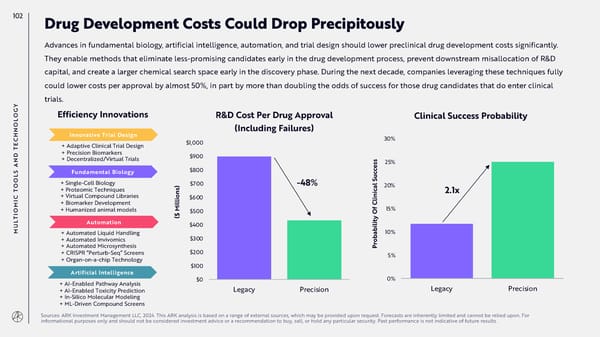
102 Drug Development Costs Could Drop Precipitously Advances in fundamental biology, artificial intelligence, automation, and trial design should lower preclinical drug development costs significantly. They enable methods that eliminate less-promising candidates early in the drug development process, prevent downstream misallocation of R&D capital, and create a larger chemical search space early in the discovery phase. During the next decade, companies leveraging these techniques fully could lower costs per approval by almost 50%, in part by more than doubling the odds of success for those drug candidates that do enter clinical Y trials. G Efficiency Innovations O R&D Cost Per Drug Approval Clinical Success Probability L O (Including Failures) HN Innovative Trial Design 30% C $1,000 E + Adaptive Clinical Trial Design T + Precision Biomarkers D + Decentralized/Virtual Trials $900 N 25% A $800 S Fundamental Biology L O + Single-Cell Biology $700 -48% O + Proteomic Techniques ) 20% T 2.1x + Virtual Compound Libraries C $600 MI + Biomarker Development Millions 15% O + Humanized animal models $500 I ($ T L Automation $400 MU + Automated Liquid Handling 10% + Automated Invivomics $300 + Automated Microsynthesis Probability Of Clinical Success + CRISPR “Perturb-Seq” Screens $200 5% + Organ-on-a-chip Technology $100 Artificial Intelligence + AI-Enabled Pathway Analysis $0 0% + AI-Enabled Toxicity Prediction Legacy Precision Legacy Precision + In-Silico Molecular Modeling + ML-Driven Compound Screens Sources: ARK Investment Management LLC, 2024. This ARK analysis is based on a range of external sources, which may be provided upon request. Forecasts are inherently limited and cannot be relied upon. For informational purposes only and should not be considered investment advice or a recommendation to buy, sell, or hold any particular security. Past performance is not indicative of future results.
 Annual Research Report | Big Ideas 2024 Page 101 Page 103
Annual Research Report | Big Ideas 2024 Page 101 Page 103