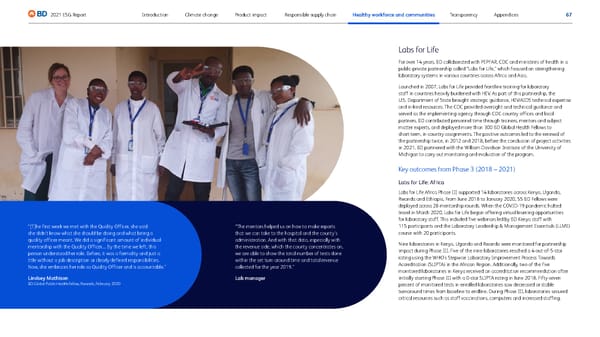
67 2021 ESG Report Transparency Responsible supply chain Product impact Climate change Introduction Healthy workforce and communities Appendices Photo: Lindsey Mathison, Rwanda, 2020 Labs for Life For over 14 years, BD collaborated with PEPFAR, CDC and ministries of health in a public-private partnership called “Labs for Life,” which focused on strengthening laboratory systems in various countries across Africa and Asia. Launched in 2007, Labs for Life provided frontline training for laboratory staff in countries heavily burdened with HIV. As part of this partnership, the U.S. Department of State brought strategic guidance, HIV/AIDS technical expertise and in-kind resources. The CDC provided oversight and technical guidance and served as the implementing agency through CDC country offices and local partners. BD contributed personnel time through trainers, mentors and subject matter experts, and deployed more than 300 BD Global Health Fellows to short-term, in-country assignments. The positive outcomes led to the renewal of the partnership twice, in 2012 and 2018, before the conclusion of project activities in 2021. BD partnered with the William Davidson Institute of the University of Michigan to carry out monitoring and evaluation of the program. Key outcomes from Phase 3 (2018 – 2021) Labs for Life: Africa Labs for Life Africa Phase III supported 14 laboratories across Kenya, Uganda, Rwanda and Ethiopia. From June 2018 to January 2020, 55 BD Fellows were deployed across 28 mentorship rounds. When the COVID-19 pandemic halted travel in March 2020, Labs for Life began offering virtual learning opportunities for laboratory staff. This included five webinars led by BD Kenya staff with 115 participants and the Laboratory Leadership & Management Essentials (LLME) course with 20 participants. Nine laboratories in Kenya, Uganda and Rwanda were monitored for partnership impact during Phase III. Five of the nine laboratories reached a 4-out-of-5-star rating using the WHO’s Stepwise Laboratory Improvement Process Towards Accreditation (SLIPTA) in the African Region. Additionally, two of the five monitored laboratories in Kenya received an accreditation recommendation after initially starting Phase III with a 0-star SLIPTA rating in June 2018. Fifty-seven percent of monitored tests in enrolled laboratories saw decreased or stable turnaround times from baseline to endline. During Phase III, laboratories secured critical resources such as staff vaccinations, computers and increased staffing. “[T]he first week we met with the Quality Officer, she said she didn’t know what she should be doing and what being a quality officer meant. We did a significant amount of individual mentorship with the Quality Officer.... By the time we left, this person understood her role. Before, it was a formality and just a title without a job description or clearly defined responsibilities. Now, she embraces her role as Quality Officer and is accountable.” Lindsey Mathison BD Global Public Health Fellow, Rwanda, February 2020 “The mentors helped us on how to make reports that we can take to the hospital and the county’s administration. And with that data, especially with the revenue side, which the county concentrates on, we are able to show the total number of tests done within the set turn around time and total revenue collected for the year 2019.” Lab manager
 BD ESG Report Page 66 Page 68
BD ESG Report Page 66 Page 68