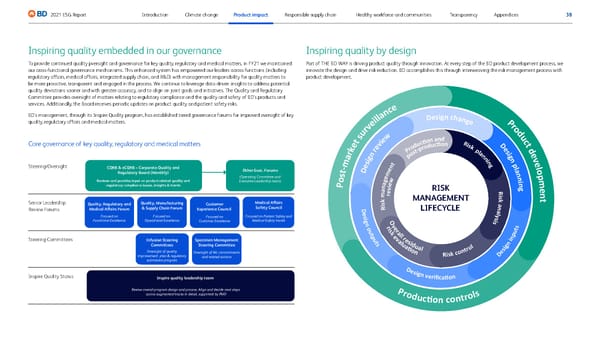
38 2021 ESG Report Appendices Transparency Healthy workforce and communities Responsible supply chain Climate change Introduction Product impact Inspiring quality embedded in our governance To provide continued quality pversight and governance for key quality, regulatory and medical matters, in FY21 we maintained our cross-functional governance mechanisms. This enhanced system has empowered our leaders across functions (including regulatory affairs, medical affairs, integrated supply chain, and R&D) with management responsibility for quality matters to be more proactive, transparent and engaged in the process. We continue to leverage data-driven insights to address potential quality deviations sooner and with greater accuracy, and to align on joint goals and initiatives. The Quality and Regulatory Committee provides oversight of matters relating to regulatory compliance and the quality and safety of BD’s products and services. Additionally, the Board receives periodic updates on product quality and patient safety risks. BD’s management, through its Inspire Quality program, has established tiered governance forums for improved oversight of key quality, regulatory affairs and medical matters. Core governance of key quality, regulatory and medical matters S teering/Oversight Senior Leade rship R eview Forums S teering Committees Inspi re Quality Status CQRB & eCQRB – Corporate Quality and Regulatory Board (Monthly) Other Exec. Forums (Operating Committee and Executive Leadership team) Inspire quality leadership team Review overall program design and process. Align and decide next steps across augmented tracks in detail, supported by PMO Reviews and provides input on product-related quality and regulatory compliance issues, insights & trends Quality, Regulatory and Medical Affairs Forum Customer Experience Council Quality, Manufacturing & Supply Chain Forum Focused on Functional Excellence Focused on Customer Excellence Specimen Management Steering Committee Oversight of WL commitments and related actions Infusion Steering Committees Oversight of quality improvement plan & regulatory submission program Focused on Operational Excellence Medical Affairs Safety Council Focused on Patient Safety and Medical Safety trends Inspiring quality by design Part of THE BD WAY is driving product quality through innovation. At every step of the BD product development process, we innovate the design and drive risk reduction. BD accomplishes this through interweaving the risk management process with product development. P o s t - m a r k e t s u r v e i l l a n c e P r o d u c t d e v e l o p m e n t P r o d u c � o n c o n t r o l s RISK MANAGEMENT LIFECYCLE D e s i g n i n p u t s D e s i g n v e r i fi c a � o n r i s k e v a l u a � o n O v e r a l l r e s i d u a l R i s k c o n t r o l D e s i g n o u t p u t s D e s i g n r e v i e w D e s i g n p l a n n i n g R i s k a n a l y s i s R i s k p l a n n i n g P r o d u c � o n a n d p o s t - p r o d u c � o n r e v i e w R i s k m a n a g e m e n t D e s i g n c h a n g e
 BD ESG Report Page 37 Page 39
BD ESG Report Page 37 Page 39