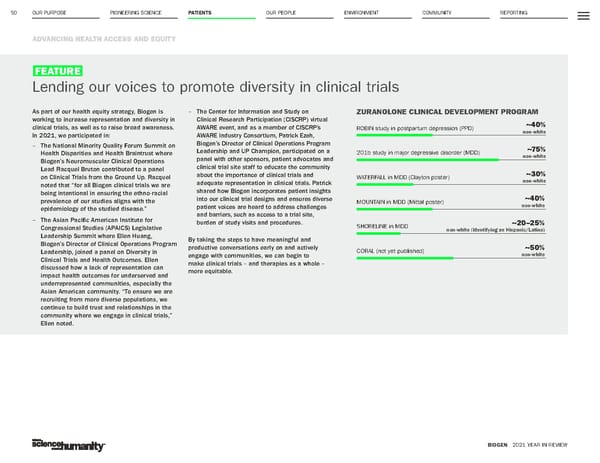
OUR PURPOSE PIONEERING SCIENCE PATIENTS OUR PEOPLE ENVIRONMENT COMMUNITY REPORTING 50 ADVANCING HEALTH ACCESS AND EQUITY BIOGEN 2021 YEAR IN REVIEW FEATURE Lending our voices to promote diversity in clinical trials As part of our health equity strategy, Biogen is working to increase representation and diversity in clinical trials, as well as to raise broad awareness. In 2021, we participated in: – The National Minority Quality Forum Summit on Health Disparities and Health Braintrust where Biogen’s Neuromuscular Clinical Operations Lead Racquel Bruton contributed to a panel on Clinical Trials from the Ground Up. Racquel noted that “for all Biogen clinical trials we are being intentional in ensuring the ethno-racial prevalence of our studies aligns with the epidemiology of the studied disease.” – The Asian Pacific American Institute for Congressional Studies (APAICS) Legislative Leadership Summit where Ellen Huang, Biogen’s Director of Clinical Operations Program Leadership, joined a panel on Diversity in Clinical Trials and Health Outcomes. Ellen discussed how a lack of representation can impact health outcomes for underserved and underrepresented communities, especially the Asian American community. “To ensure we are recruiting from more diverse populations, we continue to build trust and relationships in the community where we engage in clinical trials,” Ellen noted. ZURANOLONE CLINICAL DEVELOPMENT PROGRAM ROBIN study in postpartum depression (PPD) 201b study in major depressive disorder (MDD) WATERFALL in MDD (Clayton poster) MOUNTAIN in MDD (Mittal poster) SHORELINE in MDD CORAL (not yet published) – The Center for Information and Study on Clinical Research Participation (CISCRP) virtual AWARE event, and as a member of CISCRP’s AWARE Industry Consortium, Patrick Ezeh, Biogen’s Director of Clinical Operations Program Leadership and UP Champion, participated on a panel with other sponsors, patient advocates and clinical trial site staff to educate the community about the importance of clinical trials and adequate representation in clinical trials. Patrick shared how Biogen incorporates patient insights into our clinical trial designs and ensures diverse patient voices are heard to address challenges and barriers, such as access to a trial site, burden of study visits and procedures. By taking the steps to have meaningful and productive conversations early on and actively engage with communities, we can begin to make clinical trials – and therapies as a whole – more equitable. ~40% non-white ~75% non-white ~30% non-white ~40% non-white ~20–25% non-white (identifying as Hispanic/Latino) ~50% non-white
 Biogen Year In Review Page 49 Page 51
Biogen Year In Review Page 49 Page 51