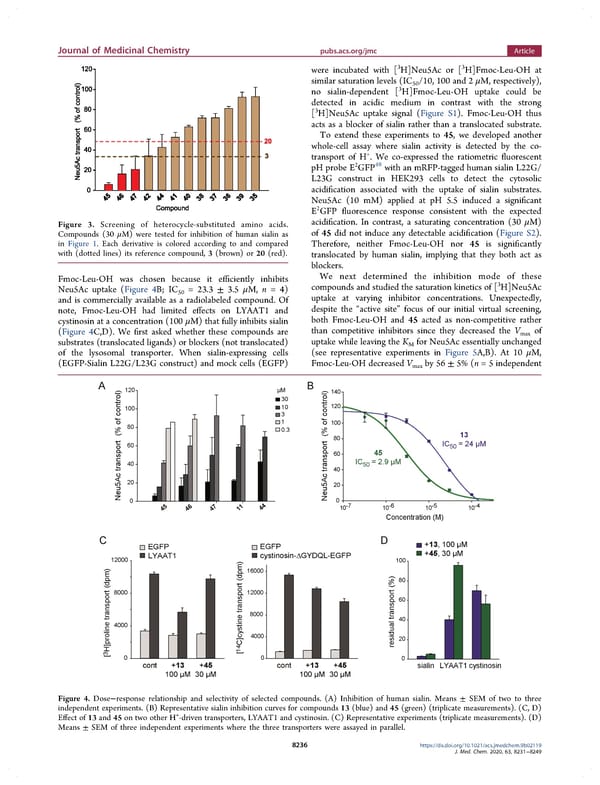
Journal of Medicinal Chemistry pubs.acs.org/jmc Article were incubated with [3 3 H]Neu5Ac or [ H]Fmoc-Leu-OH at similar saturation levels (IC /10, 100 and 2 μM, respectively), 3 50 no sialin-dependent [ H]Fmoc-Leu-OH uptake could be detected in acidic medium in contrast with the strong 3 [ H]Neu5Ac uptake signal (Figure S1). Fmoc-Leu-OH thus acts as a blocker of sialin rather than a translocated substrate. To extend these experiments to 45, we developed another whole-cell assay where sialin activity is detected by the co- + transport of H . We co-expressed the ratiometric fluorescent 2 48 pHprobeE GFP withanmRFP-tagged humansialin L22G/ L23G construct in HEK293 cells to detect the cytosolic acidification associated with the uptake of sialin substrates. Neu5Ac (10 mM) applied at pH 5.5 induced a significant 2 E GFP fluorescence response consistent with the expected Figure 3. Screening of heterocycle-substituted amino acids. acidification. In contrast, a saturating concentration (30 μM) Compounds (30 μM) were tested for inhibition of human sialin as of 45 did not induce any detectable acidification (Figure S2). in Figure 1. Each derivative is colored according to and compared Therefore, neither Fmoc-Leu-OH nor 45 is significantly with (dotted lines) its reference compound, 3 (brown) or 20 (red). translocated by human sialin, implying that they both act as blockers. Fmoc-Leu-OH was chosen because it efficiently inhibits We next determined the inhibition mode of these Neu5Ac uptake (Figure 4B; IC = 23.3 ± 3.5 μM, n =4) compounds and studied the saturation kinetics of [3H]Neu5Ac 50 uptake at varying inhibitor concentrations. Unexpectedly, and is commercially available as a radiolabeled compound. Of note, Fmoc-Leu-OH had limited effects on LYAAT1 and despite the “active site” focus of our initial virtual screening, cystinosin at a concentration (100 μM) that fully inhibits sialin both Fmoc-Leu-OH and 45 acted as non-competitive rather (Figure 4C,D). We first asked whether these compounds are than competitive inhibitors since they decreased the Vmax of substrates (translocated ligands) or blockers (not translocated) uptake while leaving the K for Neu5Ac essentially unchanged M of the lysosomal transporter. When sialin-expressing cells (see representative experiments in Figure 5A,B). At 10 μM, (EGFP-Sialin L22G/L23G construct) and mock cells (EGFP) Fmoc-Leu-OHdecreased Vmax by 56 ± 5% (n = 5 independent Figure 4. Dose−response relationship and selectivity of selected compounds. (A) Inhibition of human sialin. Means ± SEM of two to three independent experiments. (B) Representative sialin inhibition curves for compounds 13 (blue) and 45 (green) (triplicate measurements). (C, D) + Effect of 13 and 45 on two other H -driven transporters, LYAAT1 and cystinosin. (C) Representative experiments (triplicate measurements). (D) Means ± SEM of three independent experiments where the three transporters were assayed in parallel. 8236 https://dx.doi.org/10.1021/acs.jmedchem.9b02119 J. Med. Chem. 2020, 63, 8231−8249
 Amino Acids Bearing Aromatic or Heteroaromatic Substituents as a New Class of Ligands for the Lysosomal Sialic Acid Transporter Sialin Page 5 Page 7
Amino Acids Bearing Aromatic or Heteroaromatic Substituents as a New Class of Ligands for the Lysosomal Sialic Acid Transporter Sialin Page 5 Page 7