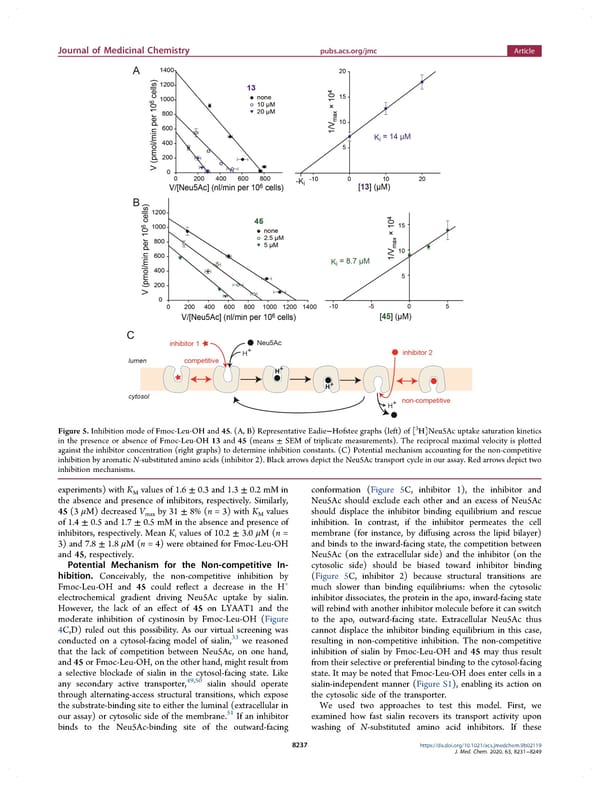
Journal of Medicinal Chemistry pubs.acs.org/jmc Article 3 Figure 5. Inhibition mode of Fmoc-Leu-OH and 45. (A, B) Representative Eadie−Hofstee graphs (left) of [ H]Neu5Ac uptake saturation kinetics in the presence or absence of Fmoc-Leu-OH 13 and 45 (means ± SEM of triplicate measurements). The reciprocal maximal velocity is plotted against the inhibitor concentration (right graphs) to determine inhibition constants. (C) Potential mechanism accounting for the non-competitive inhibition by aromatic N-substituted amino acids (inhibitor 2). Black arrows depict the Neu5Ac transport cycle in our assay. Red arrows depict two inhibition mechanisms. experiments) with K values of 1.6 ± 0.3 and 1.3 ± 0.2 mM in conformation (Figure 5C, inhibitor 1), the inhibitor and M the absence and presence of inhibitors, respectively. Similarly, Neu5Ac should exclude each other and an excess of Neu5Ac 45 (3 μM) decreased V by 31 ± 8% (n = 3) with K values should displace the inhibitor binding equilibrium and rescue max M of 1.4 ± 0.5 and 1.7 ± 0.5 mM in the absence and presence of inhibition. In contrast, if the inhibitor permeates the cell inhibitors, respectively. Mean K values of 10.2 ± 3.0 μM(n = membrane (for instance, by diffusing across the lipid bilayer) i 3) and 7.8 ± 1.8 μM(n = 4) were obtained for Fmoc-Leu-OH and binds to the inward-facing state, the competition between and 45, respectively. Neu5Ac (on the extracellular side) and the inhibitor (on the Potential Mechanism for the Non-competitive In- cytosolic side) should be biased toward inhibitor binding hibition. Conceivably, the non-competitive inhibition by (Figure 5C, inhibitor 2) because structural transitions are Fmoc-Leu-OH and 45 could reflect a decrease in the H+ much slower than binding equilibriums: when the cytosolic electrochemical gradient driving Neu5Ac uptake by sialin. inhibitor dissociates, the protein in the apo, inward-facing state However, the lack of an effect of 45 on LYAAT1 and the will rebind with another inhibitor molecule before it can switch moderate inhibition of cystinosin by Fmoc-Leu-OH (Figure to the apo, outward-facing state. Extracellular Neu5Ac thus 4C,D) ruled out this possibility. As our virtual screening was cannot displace the inhibitor binding equilibrium in this case, conducted on a cytosol-facing model of sialin,33 we reasoned resulting in non-competitive inhibition. The non-competitive that the lack of competition between Neu5Ac, on one hand, inhibition of sialin by Fmoc-Leu-OH and 45 may thus result and 45 or Fmoc-Leu-OH, on the other hand, might result from from their selective or preferential binding to the cytosol-facing a selective blockade of sialin in the cytosol-facing state. Like state. It may be noted that Fmoc-Leu-OH does enter cells in a any secondary active transporter,49,50 sialin should operate sialin-independent manner (Figure S1), enabling its action on through alternating-access structural transitions, which expose the cytosolic side of the transporter. the substrate-binding site to either the luminal (extracellular in We used two approaches to test this model. First, we 51 our assay) or cytosolic side of the membrane. If an inhibitor examined how fast sialin recovers its transport activity upon binds to the Neu5Ac-binding site of the outward-facing washing of N-substituted amino acid inhibitors. If these 8237 https://dx.doi.org/10.1021/acs.jmedchem.9b02119 J. Med. Chem. 2020, 63, 8231−8249
 Amino Acids Bearing Aromatic or Heteroaromatic Substituents as a New Class of Ligands for the Lysosomal Sialic Acid Transporter Sialin Page 6 Page 8
Amino Acids Bearing Aromatic or Heteroaromatic Substituents as a New Class of Ligands for the Lysosomal Sialic Acid Transporter Sialin Page 6 Page 8