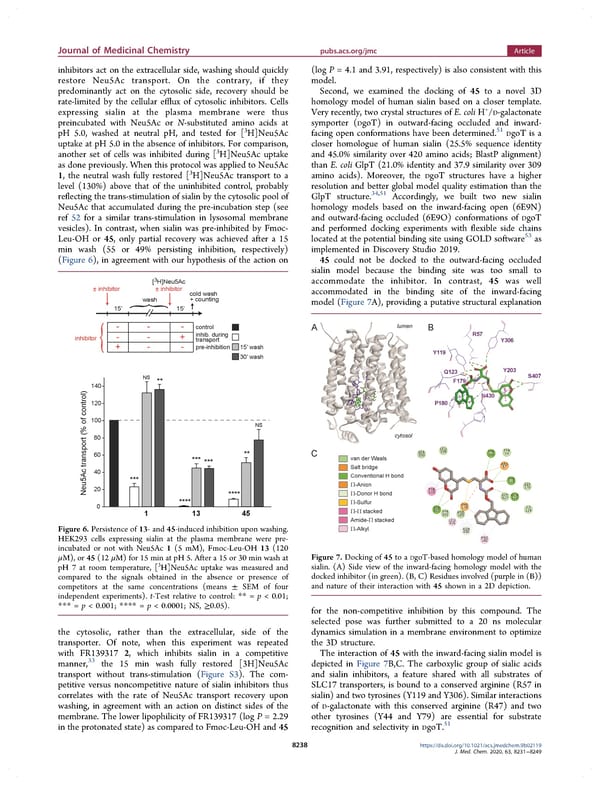
Journal of Medicinal Chemistry pubs.acs.org/jmc Article inhibitors act on the extracellular side, washing should quickly (log P = 4.1 and 3.91, respectively) is also consistent with this restore Neu5Ac transport. On the contrary, if they model. predominantly act on the cytosolic side, recovery should be Second, we examined the docking of 45 to a novel 3D rate-limited by the cellular efflux of cytosolic inhibitors. Cells homology model of human sialin based on a closer template. Very recently, two crystal structures of E. coli H+ expressing sialin at the plasma membrane were thus /D-galactonate preincubated with Neu5Ac or N-substituted amino acids at symporter (DgoT) in outward-facing occluded and inward- 3 51 pH 5.0, washed at neutral pH, and tested for [ H]Neu5Ac facing open conformations have been determined. DgoT is a uptake at pH 5.0 in the absence of inhibitors. For comparison, closer homologue of human sialin (25.5% sequence identity 3 another set of cells was inhibited during [ H]Neu5Ac uptake and 45.0% similarity over 420 amino acids; BlastP alignment) as done previously. When this protocol was applied to Neu5Ac than E. coli GlpT (21.0% identity and 37.9 similarity over 309 1, the neutral wash fully restored [3H]Neu5Ac transport to a amino acids). Moreover, the DgoT structures have a higher level (130%) above that of the uninhibited control, probably resolution and better global model quality estimation than the 34,51 reflecting the trans-stimulation of sialin by the cytosolic pool of GlpT structure. Accordingly, we built two new sialin Neu5Ac that accumulated during the pre-incubation step (see homology models based on the inward-facing open (6E9N) ref 52 for a similar trans-stimulation in lysosomal membrane and outward-facing occluded (6E9O) conformations of DgoT vesicles). In contrast, when sialin was pre-inhibited by Fmoc- and performed docking experiments with flexible side chains Leu-OH or 45, only partial recovery was achieved after a 15 53 located at the potential binding site using GOLD software as min wash (55 or 49% persisting inhibition, respectively) implemented in Discovery Studio 2019. (Figure 6), in agreement with our hypothesis of the action on 45 could not be docked to the outward-facing occluded sialin model because the binding site was too small to accommodate the inhibitor. In contrast, 45 was well accommodated in the binding site of the inward-facing model (Figure 7A), providing a putative structural explanation Figure 6. Persistence of 13- and 45-induced inhibition upon washing. HEK293 cells expressing sialin at the plasma membrane were pre- incubated or not with Neu5Ac 1 (5 mM), Fmoc-Leu-OH 13 (120 μM), or 45 (12 μM) for 15 min at pH 5. After a 15 or 30 min wash at Figure 7. Docking of 45 to a DgoT-based homology model of human 3 sialin. (A) Side view of the inward-facing homology model with the pH 7 at room temperature, [ H]Neu5Ac uptake was measured and compared to the signals obtained in the absence or presence of docked inhibitor (in green). (B, C) Residues involved (purple in (B)) competitors at the same concentrations (means ± SEM of four and nature of their interaction with 45 shown in a 2D depiction. independent experiments). t-Test relative to control: ** = p < 0.01; *** = p < 0.001; **** = p < 0.0001; NS, ≥0.05). for the non-competitive inhibition by this compound. The selected pose was further submitted to a 20 ns molecular the cytosolic, rather than the extracellular, side of the dynamics simulation in a membrane environment to optimize transporter. Of note, when this experiment was repeated the 3D structure. with FR139317 2, which inhibits sialin in a competitive The interaction of 45 with the inward-facing sialin model is manner,33 the 15 min wash fully restored [3H]Neu5Ac depicted in Figure 7B,C. The carboxylic group of sialic acids transport without trans-stimulation (Figure S3). The com- and sialin inhibitors, a feature shared with all substrates of petitive versus noncompetitive nature of sialin inhibitors thus SLC17 transporters, is bound to a conserved arginine (R57 in correlates with the rate of Neu5Ac transport recovery upon sialin) and two tyrosines (Y119 and Y306). Similar interactions washing, in agreement with an action on distinct sides of the of D-galactonate with this conserved arginine (R47) and two membrane. The lower lipophilicity of FR139317 (log P = 2.29 other tyrosines (Y44 and Y79) are essential for substrate in the protonated state) as compared to Fmoc-Leu-OH and 45 51 recognition and selectivity in DgoT. 8238 https://dx.doi.org/10.1021/acs.jmedchem.9b02119 J. Med. Chem. 2020, 63, 8231−8249
 Amino Acids Bearing Aromatic or Heteroaromatic Substituents as a New Class of Ligands for the Lysosomal Sialic Acid Transporter Sialin Page 7 Page 9
Amino Acids Bearing Aromatic or Heteroaromatic Substituents as a New Class of Ligands for the Lysosomal Sialic Acid Transporter Sialin Page 7 Page 9