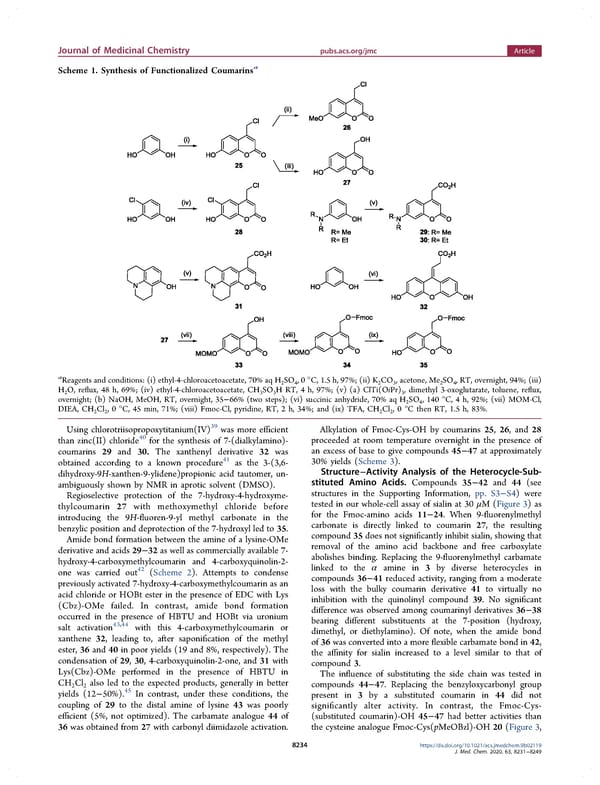
Journal of Medicinal Chemistry pubs.acs.org/jmc Article Scheme 1. Synthesis of Functionalized Coumarinsa a Reagents and conditions: (i) ethyl-4-chloroacetoacetate, 70% aq H SO ,0°C, 1.5 h, 97%; (ii) K CO , acetone, Me SO , RT, overnight, 94%; (iii) 2 4 2 3 2 4 HO,reflux, 48 h, 69%; (iv) ethyl-4-chloroacetoacetate, CH SO H RT, 4 h, 97%; (v) (a) ClTi(OiPr) , dimethyl 3-oxoglutarate, toluene, reflux, 2 3 3 3 overnight; (b) NaOH, MeOH, RT, overnight, 35−66% (two steps); (vi) succinic anhydride, 70% aq H SO , 140 °C, 4 h, 92%; (vii) MOM-Cl, 2 4 DIEA, CH Cl ,0°C, 45 min, 71%; (viii) Fmoc-Cl, pyridine, RT, 2 h, 34%; and (ix) TFA, CH Cl ,0°C then RT, 1.5 h, 83%. 2 2 2 2 Using chlorotriisopropoxytitanium(IV)39 was more efficient Alkylation of Fmoc-Cys-OH by coumarins 25, 26, and 28 than zinc(II) chloride40 for the synthesis of 7-(dialkylamino)- proceeded at room temperature overnight in the presence of coumarins 29 and 30. The xanthenyl derivative 32 was an excess of base to give compounds 45−47 at approximately 41 30% yields (Scheme 3). obtained according to a known procedure as the 3-(3,6- Structure−Activity Analysis of the Heterocycle-Sub- dihydroxy-9H-xanthen-9-ylidene)propionic acid tautomer, un- stituted Amino Acids. Compounds 35−42 and 44 (see ambiguously shown by NMR in aprotic solvent (DMSO). structures in the Supporting Information, pp. S3−S4) were Regioselective protection of the 7-hydroxy-4-hydroxyme- tested in our whole-cell assay of sialin at 30 μM(Figure 3)as thylcoumarin 27 with methoxymethyl chloride before for the Fmoc-amino acids 11−24. When 9-fluorenylmethyl introducing the 9H-fluoren-9-yl methyl carbonate in the carbonate is directly linked to coumarin 27, the resulting benzylic position and deprotection of the 7-hydroxyl led to 35. compound 35 does not significantly inhibit sialin, showing that Amide bond formation between the amine of a lysine-OMe removal of the amino acid backbone and free carboxylate derivative and acids 29−32 as well as commercially available 7- abolishes binding. Replacing the 9-fluorenylmethyl carbamate hydroxy-4-carboxymethylcoumarin and 4-carboxyquinolin-2- linked to the α amine in 3 by diverse heterocycles in one was carried out42 (Scheme 2). Attempts to condense previously activated 7-hydroxy-4-carboxymethylcoumarin as an compounds 36−41 reduced activity, ranging from a moderate acid chloride or HOBt ester in the presence of EDC with Lys loss with the bulky coumarin derivative 41 to virtually no (Cbz)-OMe failed. In contrast, amide bond formation inhibition with the quinolinyl compound 39. No significant occurred in the presence of HBTU and HOBt via uronium difference was observed among coumarinyl derivatives 36−38 43,44 with this 4-carboxymethylcoumarin or bearing different substituents at the 7-position (hydroxy, salt activation dimethyl, or diethylamino). Of note, when the amide bond xanthene 32, leading to, after saponification of the methyl of 36 was converted into a more flexible carbamate bond in 42, ester, 36 and 40 in poor yields (19 and 8%, respectively). The the affinity for sialin increased to a level similar to that of condensation of 29, 30, 4-carboxyquinolin-2-one, and 31 with compound 3. Lys(Cbz)-OMe performed in the presence of HBTU in The influence of substituting the side chain was tested in CHCl also led to the expected products, generally in better compounds 44−47. Replacing the benzyloxycarbonyl group 2 2 45 yields (12−50%). In contrast, under these conditions, the present in 3 by a substituted coumarin in 44 did not coupling of 29 to the distal amine of lysine 43 was poorly significantly alter activity. In contrast, the Fmoc-Cys- efficient (5%, not optimized). The carbamate analogue 44 of (substituted coumarin)-OH 45−47 had better activities than 36 was obtained from 27 with carbonyl diimidazole activation. the cysteine analogue Fmoc-Cys(pMeOBzl)-OH 20 (Figure 3, 8234 https://dx.doi.org/10.1021/acs.jmedchem.9b02119 J. Med. Chem. 2020, 63, 8231−8249
 Amino Acids Bearing Aromatic or Heteroaromatic Substituents as a New Class of Ligands for the Lysosomal Sialic Acid Transporter Sialin Page 3 Page 5
Amino Acids Bearing Aromatic or Heteroaromatic Substituents as a New Class of Ligands for the Lysosomal Sialic Acid Transporter Sialin Page 3 Page 5