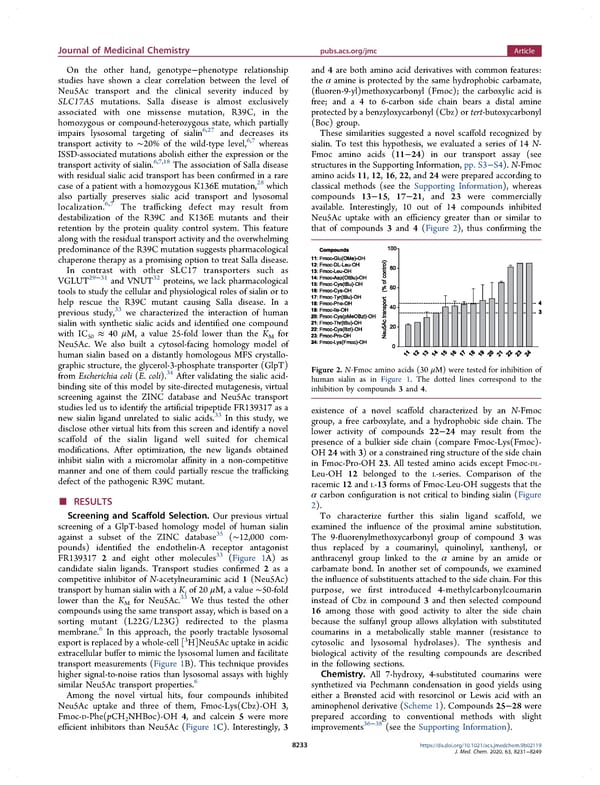
Journal of Medicinal Chemistry pubs.acs.org/jmc Article On the other hand, genotype−phenotype relationship and 4 are both amino acid derivatives with common features: studies have shown a clear correlation between the level of the α amine is protected by the same hydrophobic carbamate, Neu5Ac transport and the clinical severity induced by (fluoren-9-yl)methoxycarbonyl (Fmoc); the carboxylic acid is SLC17A5 mutations. Salla disease is almost exclusively free; and a 4 to 6-carbon side chain bears a distal amine associated with one missense mutation, R39C, in the protected by a benzyloxycarbonyl (Cbz) or tert-butoxycarbonyl homozygous or compound-heterozygous state, which partially (Boc) group. impairs lysosomal targeting of sialin6,27 and decreases its These similarities suggested a novel scaffold recognized by transport activity to ∼20% of the wild-type level,6,7 whereas sialin. To test this hypothesis, we evaluated a series of 14 N- ISSD-associated mutations abolish either the expression or the Fmoc amino acids (11−24) in our transport assay (see 6,7,18 structures in the Supporting Information, pp. S3−S4). N-Fmoc transport activity of sialin. The association of Salla disease with residual sialic acid transport has been confirmed in a rare amino acids 11, 12, 16, 22, and 24 were prepared according to 28 classical methods (see the Supporting Information), whereas case of a patient with a homozygous K136E mutation, which also partially preserves sialic acid transport and lysosomal compounds 13−15, 17−21,and23 were commercially localization.6,7 The trafficking defect may result from available. Interestingly, 10 out of 14 compounds inhibited destabilization of the R39C and K136E mutants and their Neu5Ac uptake with an efficiency greater than or similar to retention by the protein quality control system. This feature that of compounds 3 and 4 (Figure 2), thus confirming the along with the residual transport activity and the overwhelming predominance of the R39C mutation suggests pharmacological chaperone therapy as a promising option to treat Salla disease. In contrast with other SLC17 transporters such as 29−31 32 VGLUT and VNUT proteins, we lack pharmacological tools to study the cellular and physiological roles of sialin or to help rescue the R39C mutant causing Salla disease. In a 33 previous study, we characterized the interaction of human sialin with synthetic sialic acids and identified one compound with IC ≈ 40 μM, a value 25-fold lower than the K for 50 M Neu5Ac. We also built a cytosol-facing homology model of human sialin based on a distantly homologous MFS crystallo- graphic structure, the glycerol-3-phosphate transporter (GlpT) Figure 2. N-Fmoc amino acids (30 μM) were tested for inhibition of 34 from Escherichia coli (E. coli). After validating the sialic acid- human sialin as in Figure 1. The dotted lines correspond to the binding site of this model by site-directed mutagenesis, virtual inhibition by compounds 3 and 4. screening against the ZINC database and Neu5Ac transport studies led us to identify the artificial tripeptide FR139317 as a existence of a novel scaffold characterized by an N-Fmoc 33 new sialin ligand unrelated to sialic acids. In this study, we group, a free carboxylate, and a hydrophobic side chain. The disclose other virtual hits from this screen and identify a novel lower activity of compounds 22−24 may result from the scaffold of the sialin ligand well suited for chemical presence of a bulkier side chain (compare Fmoc-Lys(Fmoc)- modifications. After optimization, the new ligands obtained OH24with3)oraconstrainedringstructure of the side chain inhibit sialin with a micromolar affinity in a non-competitive in Fmoc-Pro-OH 23. All tested amino acids except Fmoc-DL- manner and one of them could partially rescue the trafficking Leu-OH 12 belonged to the L-series. Comparison of the defect of the pathogenic R39C mutant. racemic 12 and L-13 forms of Fmoc-Leu-OH suggests that the RESULTS α carbon configuration is not critical to binding sialin (Figure ■ 2). Screening and Scaffold Selection. Our previous virtual To characterize further this sialin ligand scaffold, we screening of a GlpT-based homology model of human sialin examined the influence of the proximal amine substitution. 35 The 9-fluorenylmethoxycarbonyl group of compound 3 was against a subset of the ZINC database (∼12,000 com- pounds) identified the endothelin-A receptor antagonist thus replaced by a coumarinyl, quinolinyl, xanthenyl, or FR139317 2 and eight other molecules33 (Figure 1A) as anthracenyl group linked to the α amine by an amide or candidate sialin ligands. Transport studies confirmed 2 as a carbamate bond. In another set of compounds, we examined competitive inhibitor of N-acetylneuraminic acid 1 (Neu5Ac) the influence of substituents attached to the side chain. For this transport by human sialin with a Ki of 20 μM, a value ∼50-fold purpose, we first introduced 4-methylcarbonylcoumarin lower than the K for Neu5Ac.33 We thus tested the other instead of Cbz in compound 3 and then selected compound M compoundsusingthesametransport assay, which is based on a 16 among those with good activity to alter the side chain sorting mutant (L22G/L23G) redirected to the plasma because the sulfanyl group allows alkylation with substituted 6 coumarins in a metabolically stable manner (resistance to membrane. In this approach, the poorly tractable lysosomal 3 cytosolic and lysosomal hydrolases). The synthesis and export is replaced by a whole-cell [ H]Neu5Ac uptake in acidic extracellular buffer to mimic the lysosomal lumen and facilitate biological activity of the resulting compounds are described transport measurements (Figure 1B). This technique provides in the following sections. higher signal-to-noise ratios than lysosomal assays with highly Chemistry. All 7-hydroxy, 4-substituted coumarins were similar Neu5Ac transport properties.6 synthetized via Pechmann condensation in good yields using Among the novel virtual hits, four compounds inhibited either a Brønsted acid with resorcinol or Lewis acid with an Neu5Ac uptake and three of them, Fmoc-Lys(Cbz)-OH 3, aminophenol derivative (Scheme 1). Compounds 25−28 were Fmoc-D-Phe(pCH NHBoc)-OH 4, and calcein 5 were more prepared according to conventional methods with slight 2 36−38 efficient inhibitors than Neu5Ac (Figure 1C). Interestingly, 3 improvements (see the Supporting Information). 8233 https://dx.doi.org/10.1021/acs.jmedchem.9b02119 J. Med. Chem. 2020, 63, 8231−8249
 Amino Acids Bearing Aromatic or Heteroaromatic Substituents as a New Class of Ligands for the Lysosomal Sialic Acid Transporter Sialin Page 2 Page 4
Amino Acids Bearing Aromatic or Heteroaromatic Substituents as a New Class of Ligands for the Lysosomal Sialic Acid Transporter Sialin Page 2 Page 4