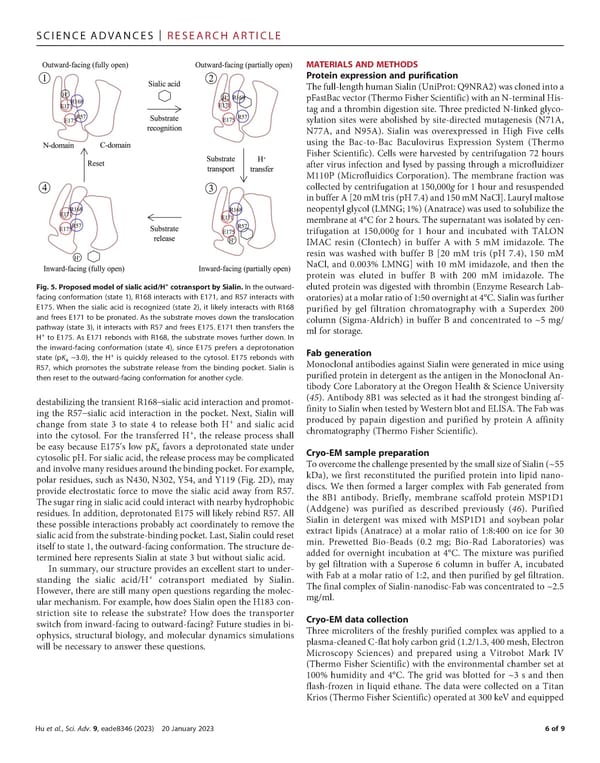
SCIENCEADVANCES | RESEARCHARTICLE MATERIALSANDMETHODS Protein expression and puri昀椀cation Thefull-lengthhumanSialin(UniProt:Q9NRA2)wasclonedintoa pFastBacvector(ThermoFisherScientific)withanN-terminalHis- tag and a thrombin digestion site. Three predicted N-linked glyco- sylation sites were abolished by site-directed mutagenesis (N71A, N77A, and N95A). Sialin was overexpressed in High Five cells using the Bac-to-Bac Baculovirus Expression System (Thermo Fisher Scientific). Cells were harvested by centrifugation 72 hours after virus infection and lysed by passing through a microfluidizer M110P (Microfluidics Corporation). The membrane fraction was collected by centrifugation at 150,000g for 1 hour and resuspended inbufferA[20mMtris(pH7.4)and150mMNaCl].Laurylmaltose neopentylglycol(LMNG;1%)(Anatrace)wasusedtosolubilizethe membraneat4°Cfor2hours.Thesupernatantwasisolatedbycen- trifugation at 150,000g for 1 hour and incubated with TALON IMAC resin (Clontech) in buffer A with 5 mM imidazole. The resin was washed with buffer B [20 mM tris (pH 7.4), 150 mM NaCl, and 0.003% LMNG] with 10 mM imidazole, and then the protein was eluted in buffer B with 200 mM imidazole. The Fig. 5. Proposed model of sialic acid/H+ cotransport by Sialin. In the outward- eluted protein was digested with thrombin (Enzyme Research Lab- facing conformation (state 1), R168 interacts with E171, and R57 interacts with oratories)atamolarratioof1:50overnightat4°C.Sialinwasfurther E175. When the sialic acid is recognized (state 2), it likely interacts with R168 purified by gel filtration chromatography with a Superdex 200 and frees E171 to be pronated. As the substrate moves down the translocation column (Sigma-Aldrich) in buffer B and concentrated to ~5 mg/ pathway (state 3), it interacts with R57 and frees E175. E171 then transfers the ml for storage. H+ to E175. As E171 rebonds with R168, the substrate moves further down. In the inward-facing conformation (state 4), since E175 prefers a deprotonation Fab generation state (pK ~3.0), the H+ is quickly released to the cytosol. E175 rebonds with a Monoclonal antibodies against Sialin were generated in mice using R57, which promotes the substrate release from the binding pocket. Sialin is purified protein in detergent as the antigen in the Monoclonal An- then reset to the outward-facing conformation for another cycle. tibody Core Laboratory at the Oregon Health & Science University destabilizing the transient R168–sialic acid interaction and promot- (45). Antibody 8B1 was selected as it had the strongest binding af- ing the R57–sialic acid interaction in the pocket. Next, Sialin will finity to Sialin when tested by Western blot andELISA.TheFabwas change from state 3 to state 4 to release both H+ and sialic acid produced by papain digestion and purified by protein A affinity + chromatography (Thermo Fisher Scientific). into the cytosol. For the transferred H , the release process shall be easy because E175’s low pK favors a deprotonated state under a Cryo-EM sample preparation cytosolic pH. For sialic acid, the release process may be complicated ToovercomethechallengepresentedbythesmallsizeofSialin(~55 andinvolvemanyresiduesaroundthebindingpocket.Forexample, kDa), we first reconstituted the purified protein into lipid nano- polar residues, such as N430, N302, Y54, and Y119 (Fig. 2D), may discs. We then formed a larger complex with Fab generated from provide electrostatic force to move the sialic acid away from R57. the 8B1 antibody. Briefly, membrane scaffold protein MSP1D1 Thesugarringinsialicacidcouldinteractwithnearbyhydrophobic (Addgene) was purified as described previously (46). Purified residues. In addition, deprotonated E175 will likely rebind R57. All Sialin in detergent was mixed with MSP1D1 and soybean polar these possible interactions probably act coordinately to remove the extract lipids (Anatrace) at a molar ratio of 1:8:400 on ice for 30 sialic acid from the substrate-binding pocket. Last, Sialin could reset min. Prewetted Bio-Beads (0.2 mg; Bio-Rad Laboratories) was itself to state 1, the outward-facing conformation. The structure de- added for overnight incubation at 4°C. The mixture was purified termined here represents Sialin at state 3 but without sialic acid. by gel filtration with a Superose 6 column in buffer A, incubated In summary, our structure provides an excellent start to under- with Fab at a molar ratio of 1:2, and then purified by gel filtration. + standing the sialic acid/H cotransport mediated by Sialin. Thefinal complex of Sialin-nanodisc-Fab was concentrated to ~2.5 However, there are still many open questions regarding the molec- mg/ml. ular mechanism. Forexample, how does Sialin open the H183 con- striction site to release the substrate? How does the transporter Cryo-EM data collection switch from inward-facing to outward-facing? Future studies in bi- Three microliters of the freshly purified complex was applied to a ophysics, structural biology, and molecular dynamics simulations plasma-cleanedC-flatholycarbongrid(1.2/1.3,400mesh,Electron will be necessary to answer these questions. Microscopy Sciences) and prepared using a Vitrobot Mark IV (Thermo Fisher Scientific) with the environmental chamber set at 100% humidity and 4°C. The grid was blotted for ~3 s and then flash-frozen in liquid ethane. The data were collected on a Titan Krios (ThermoFisherScientific) operated at 300 keVand equipped Huetal., Sci. Adv. 9, eade8346 (2023) 20 January 2023 6of9
 The molecular mechanism of sialic acid transport mediated by Sialin Page 5 Page 7
The molecular mechanism of sialic acid transport mediated by Sialin Page 5 Page 7