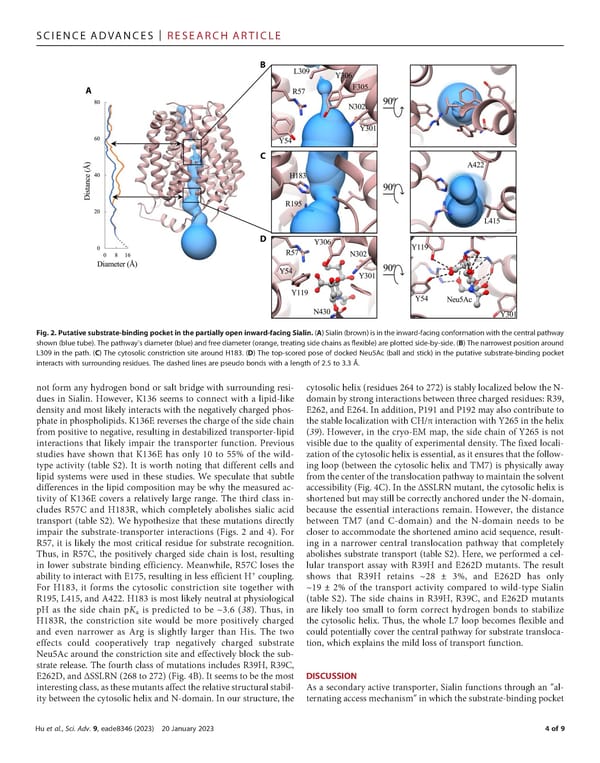
SCIENCEADVANCES | RESEARCHARTICLE Fig. 2. Putativesubstrate-bindingpocketinthepartiallyopeninward-facingSialin.(A)Sialin(brown)isintheinward-facingconformationwiththecentralpathway shown(bluetube).Thepathway’sdiameter(blue)andfreediameter(orange,treatingsidechainsas昀氀exible)areplottedside-by-side.(B)Thenarrowestpositionaround L309 in the path. (C) The cytosolic constriction site around H183. (D) The top-scored pose of docked Neu5Ac (ball and stick) in the putative substrate-binding pocket interacts with surrounding residues. The dashed lines are pseudo bonds with a length of 2.5 to 3.3 Å. not form any hydrogen bond or salt bridge with surrounding resi- cytosolic helix (residues 264 to 272) is stably localized below the N- dues in Sialin. However, K136 seems to connect with a lipid-like domainbystronginteractionsbetweenthreechargedresidues:R39, density and most likely interacts with the negatively charged phos- E262, and E264. In addition, P191 and P192 may also contribute to phate in phospholipids. K136E reversesthe charge of the side chain the stable localization with CH/π interaction with Y265 in the helix frompositive to negative, resulting in destabilized transporter-lipid (39). However, in the cryo-EM map, the side chain of Y265 is not interactions that likely impair the transporter function. Previous visible due to the quality of experimental density. The fixed locali- studies have shown that K136E has only 10 to 55% of the wild- zation of the cytosolic helix is essential, as it ensures that the follow- type activity (table S2). It is worth noting that different cells and ing loop (between the cytosolic helix and TM7) is physically away lipid systems were used in these studies. We speculate that subtle fromthecenterofthetranslocationpathwaytomaintainthesolvent differences in the lipid composition may be why the measured ac- accessibility (Fig. 4C). In the ΔSSLRN mutant, the cytosolic helix is tivity of K136E covers a relatively large range. The third class in- shortened but maystill be correctly anchored under the N-domain, cludes R57C and H183R, which completely abolishes sialic acid because the essential interactions remain. However, the distance transport (table S2). We hypothesize that these mutations directly between TM7 (and C-domain) and the N-domain needs to be impair the substrate-transporter interactions (Figs. 2 and 4). For closer to accommodate the shortened amino acid sequence, result- R57, it is likely the most critical residue for substrate recognition. ing in a narrower central translocation pathway that completely Thus, in R57C, the positively charged side chain is lost, resulting abolishes substrate transport (table S2). Here, we performed a cel- in lower substrate binding efficiency. Meanwhile, R57C loses the lular transport assay with R39H and E262D mutants. The result + ability to interact with E175, resulting in less efficient H coupling. shows that R39H retains ~28 ± 3%, and E262D has only For H183, it forms the cytosolic constriction site together with ~19 ± 2% of the transport activity compared to wild-type Sialin R195, L415, and A422. H183 is most likely neutral at physiological (table S2). The side chains in R39H, R39C, and E262D mutants pH as the side chain pKa is predicted to be ~3.6 (38). Thus, in are likely too small to form correct hydrogen bonds to stabilize H183R, the constriction site would be more positively charged the cytosolic helix. Thus, the whole L7 loop becomes flexible and and even narrower as Arg is slightly larger than His. The two could potentially cover the central pathway for substrate transloca- effects could cooperatively trap negatively charged substrate tion, which explains the mild loss of transport function. Neu5Acaround the constriction site and effectively block the sub- strate release. The fourth class of mutations includes R39H, R39C, E262D,andΔSSLRN(268to272)(Fig.4B).Itseemstobethemost DISCUSSION interestingclass,asthesemutantsaffecttherelativestructuralstabil- As a secondary active transporter, Sialin functions through an “al- ity between the cytosolic helix and N-domain. In our structure, the ternating access mechanism” in which the substrate-binding pocket Huetal., Sci. Adv. 9, eade8346 (2023) 20 January 2023 4of9
 The molecular mechanism of sialic acid transport mediated by Sialin Page 3 Page 5
The molecular mechanism of sialic acid transport mediated by Sialin Page 3 Page 5